Abstract
Background Acute graft-vs-host disease (aGvHD) is a major complication of allogeneic stem cell transplantation. While corticosteroids (CS) constitute the first-line (1L) therapy in aGvHD, only 30-50% of patients (pts) respond to 1L treatment. In the phase 3 REACH2 study, ruxolitinib (RUX) demonstrated superior overall response rates (ORR) vs best available therapy in pts ≥12 years with steroid-refractory (SR) aGvHD. Here, we present the first data from REACH4 (NCT03491215), a phase 1/2 open-label, single-arm, multicenter study of RUX added to CS in pediatric pts with grade II-IV treatment-naïve or SR aGvHD.
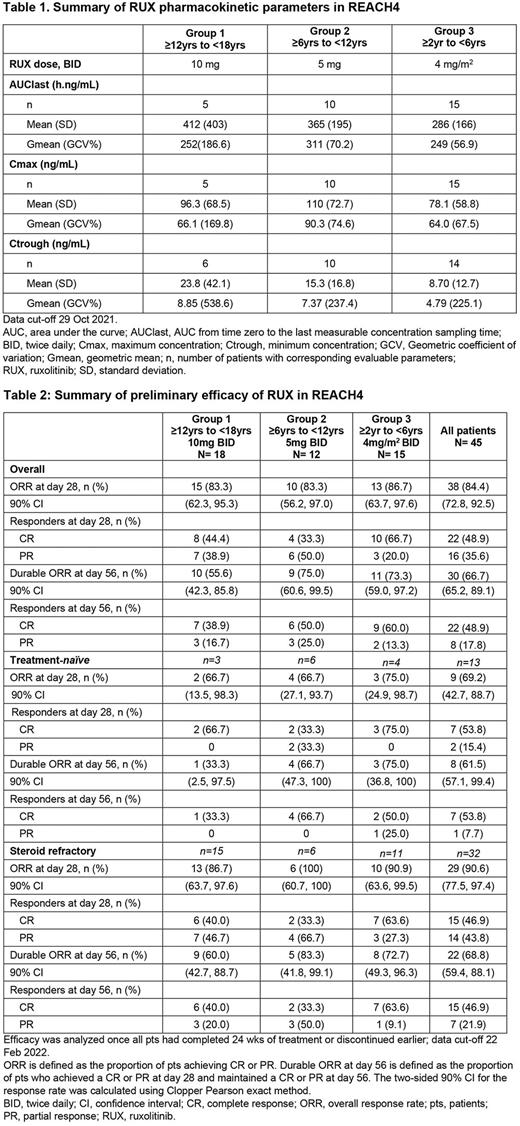
Methods REACH4 had 4 age-based treatment groups (Group 1: ≥12 to <18 years, Group 2: ≥6 to <12 years, Group 3: ≥2 to <6 years and Group 4: ≥28 days to<2 years). All pts received RUX (either a 5mg tablet or oral pediatric formulation) plus CS ± calcineurin inhibitor for 24 weeks or until discontinuation. Group 1 adolescent pts received RUX 10 mg twice daily (BID) and preliminary starting doses (predicted by physiologically based PK [PBPK]) for Groups 2 and 3 were 5 mg BID and 4 mg/m2 BID, respectively. The phase 1 primary objective was to assess pharmacokinetic (PK) parameters of RUX and define an age-appropriate Recommended Phase 2 Dose (RP2D) for Groups 2-4. The phase 2 primary objective was to measure the activity of RUX as assessed by ORR at day 28; secondary objective was to assess the rate of durable ORR at day 56. RUX taper could start at day 56 for pts with a day 28 response. PK data were analyzed at the time of confirming the RP2D; all other data were analyzed once all pts had completed 24 weeks of treatment or discontinued earlier (data cut-off 22 February 2022).
Results Overall, 45 pts were treated with RUX; 62.2% were male and the median age was 7.6 years (range, 2.3-17.4). Mean (SD) weight at baseline was 30.9 (17.5) kg. At the start of study treatment, 64.4%, 26.7% and 8.9% of pts had grade II, III and IV aGvHD, respectively; 71.1% were SR and 28.9% were treatment-naïve. At study start, the mean CS dose in all pts was 1.9 (SD 0.91) mg/kg/day. The median duration of RUX exposure was 117 days (range 8.0-342.0). At week 24, 22/45 (48.9%) pts had completed the treatment period; 30 (66.7%) pts entered long-term follow-up (up to month 24).
RUX exposure (AUClast and Cmax) were similar across age groups; the RUX starting doses were confirmed as the RP2Ds for Groups 2 and 3 (Table 1); Group 4 RP2D has not been established yet.
In preliminary efficacy analysis, the ORR in all pts was 84.4% (38/45) at day 28, with a durable ORR at day 56 of 66.7% (30/45; Table 2). ORR at day 28 and durable ORR at day 56 among treatment-naïve pts were 69.2% (9/13) and 61.5% (8/13), respectively; and among SR pts, 90.6% (29/32) and 68.8% (22/32), respectively.
In total, 38 (84.4%) pts had at least one dose change or interruption; most common reasons included adverse event (AE; 51.1%), dose tapering permitted per protocol (44.4%) and per-protocol dose change/interruption (31.1%). Overall, 23 (51.1%) pts discontinued treatment due to lack of efficacy (26.7%), AE (22.2%) and disease relapse (2.2%).
No new safety signals were observed, and the most frequently reported AEs were in line with those previously observed in RUX clinical trials. There were no confirmed cases of graft failure.
Conclusions The RP2D of RUX of 5 mg and 4 mg/m2 BID, as predicted by PBPK, were confirmed in Groups 2 and 3, respectively. RUX treatment led to high ORR, which are comparable to those previously reported in REACH2 (Zeiser et al., N Engl J Med, 2020) and from retrospective studies of RUX-treated pediatric pts (Uygun et al., Pediatr Blood Cancer, 2020; Laisne et al., Pediatr Blood Cancer, 2020). The safety profile of RUX was also consistent with that expected for RUX in this population.
This study was sponsored by Novartis Pharmaceuticals.
Disclosures
Locatelli:Amgen: Speakers Bureau; Neovii: Speakers Bureau; Medac: Speakers Bureau; BlueBird bio: Speakers Bureau; Miltenyi: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; SOBI: Speakers Bureau; Jazz Pharmaceuticals: Honoraria. Kang:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bittencourt:Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Speakers Bureau. Cleary:Novartis: Current Employment. Rosko:Novartis: Current Employment. Roussou:Novartis: Current Employment. St. Pierre:Novartis: Current Employment. Prahallad:Novartis: Current Employment. Díaz-de-Heredia:MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Biotest: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses .
OffLabel Disclosure:
Ruxolitinib, approved for the treatment of graft steroid-refractory acute graft-versus-host disease in adult and pediatric patients 12 years and older and, chronic graft-versus-host disease after failure of one or two lines of systemic therapy in adult and pediatric patients 12 years and older. Data from Phase I/II clinical trial of ruxolitinib in pediatric patients with treatment-naïve or steroid-refractory acute graft-versus-host disease will be presented.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal